In the era following the advancement of drug research, artificial intelligence (AI) has emerged as a leading force in pharmaceutical development. In recent years, the pharmaceutical industry has witnessed a significant shift towards digitization of data. However, harnessing this data to address clinical challenges has posed a notable hurdle. This challenge has spurred the adoption of artificial intelligence, which offers enhanced automation to manage vast amounts of data effectively. AI, a technology-driven system comprising various advanced tools and networks, can mimic human intelligence without posing a threat to human existence. It utilizes systems and software capable of interpreting and learning from input data to autonomously work towards specific goals. The integration of artificial intelligence in medicine continues to expand, marking a promising frontier.
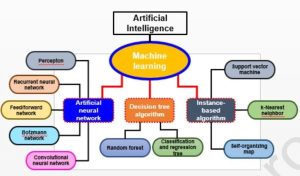
Artificial intelligence encompasses various methodological areas, including reasoning, knowledge representation, and solution searching, with machine learning (ML) serving as a fundamental paradigm. Deep learning (DL), a subset of ML, employs artificial neural networks (ANNs) that simulate the transmission of electrical impulses in the human brain, resembling human perceptions. Neural networks come in diverse types, such as Multilayer Perceptrons (MLP), Recursive Neural Networks (RNNs), and Convolutional Neural Networks (CNNs). More complex forms include Kohonen networks, RBF networks, LVQ networks, backpropagation networks, and ADALINE networks. The following diagram provides a concise overview of methodological domains in artificial intelligence.
The process of drug discovery and development, which could span over a decade and cost an average of $2.8 billion, poses formidable challenges. Despite this investment, a staggering 90% of therapeutic molecules fail to advance beyond Phase II clinical trials and regulatory approval. Algorithms like nearest neighbor, RF, extreme learning, SVMs, and deep neural networks (DNNs) offer virtual screening (VS) capabilities based on synthetic feasibility, while also predicting in vivo activity and toxicity. Some large biopharmaceutical companies have collaborated with IT companies to develop artificial intelligence platforms dedicated to discovering treatments, particularly in areas like oncology and cardiovascular diseases.
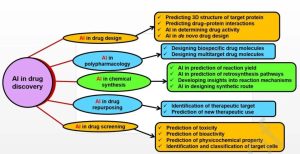
Predicting the physicochemical properties of drugs, such as solubility, distribution coefficient (logP), ionization, and intrinsic permeability, is crucial as they indirectly affect the pharmacokinetics of a drug and its targeting receptors. Various AI tools aid in predicting these properties, with ML using large datasets generated during compound optimization to train programs. Drug design algorithms include molecular descriptors, potential energy calculations, electron density analysis, and three-dimensional atomic coordinates, generating viable molecules through DNNs to predict their properties.
The efficacy of drug molecules depends on their affinity to target proteins or receptors. Molecules lacking interaction or affinity towards target proteins fail to elicit therapeutic responses. In some cases, developed drug molecules may interact with unintended proteins or receptors, leading to toxicity. Hence, predicting drug-target binding affinity (DTBA) plays a crucial role in anticipating interactions between drugs and their targets. AI-based methods measure the binding affinity of drugs by analyzing properties or similarities between the drug and its target. Network applications like ChemMapper and Similarity Ensemble Approach (SEA) are used for predicting drug-target interactions. Many strategies involving ML and DL have been employed to determine DTBA, such as KronRLS, SimBoost, DeepDTA, and PADME. ML-based approaches like KronRLS assess similarity between drug and protein molecules to determine DTBA. Similarly, SimBoost uses regression trees to predict DTBA, considering both feature-based and similarity-based interactions.
Predicting the toxicity of drug molecules is crucial to avoiding adverse effects. Cell-based in vitro tests are usually used as preliminary studies, followed by animal studies to determine compound toxicity, which increases the cost of drug discovery. Web-based tools like LimTox, pkCSM, admetSAR, and Toxtree can help reduce costs. Advanced AI-based methods look for similarities among compounds or predict compound toxicity based on input features. The Tox21 Data Challenge, organized by the National Institutes of Health, Environmental Protection Agency (EPA), and Food and Drug Administration (FDA), is an initiative to evaluate several computational techniques for predicting the toxicity of 12,707 environmental compounds and drugs. The ML algorithm DeepTox stood out by identifying static and dynamic features within molecular chemical descriptors, such as molecular weight (MW) and van der Waals forces, and could effectively predict molecular toxicity based on predefined toxicophore features.
AI Assists in Drug Design
AI assists in drug design from predicting target protein structures, crucial for designing drug molecules, to predicting drug-protein interactions essential for therapeutic success. Predicting drug-target interactions is also used to repurpose existing drugs and avoid polypharmacology. Repurposing existing drugs can directly proceed to Phase II clinical trials, reducing costs compared to developing new drug entities. “Guilty association” methods predict innovative associations between drugs and diseases, using knowledge or computation-driven networks. In computation-driven networks, ML methods employing techniques like support vectors, neural networks, logistic regression, and DL are broadly applied.
Predicting drug-protein interactions also anticipates polypharmacology opportunities, the trend of drug molecules interacting with multiple receptors, producing off-target adverse reactions. AI can design new molecules based on the principles of polypharmacology, helping to produce safer drug molecules. AI platforms like SOM, combined with extensive existing databases, can link several compounds to numerous targets and off-targets. Bayesian classifiers and SEA algorithms are used to establish connections between drug pharmacological characteristics and their potential targets.
De novo drug design methods have been widely applied to drug molecule design over the past years, with traditional de novo design methods being replaced by evolving DL methods due to their complexity and difficulty in predicting new molecule bioactivity. Researchers like Popova have developed reinforcement learning for de novo drug synthesis, including generative and predictive DNNs to develop new compounds. Similarly, generative AI models have been utilized to design molecules with ideal therapeutic effects without the need for complex rules. AI’s involvement in molecule design is beneficial for the pharmaceutical industry, offering advantages like providing online learning and simultaneous optimization of already learned data, as well as suggesting possible synthesis routes for compounds, thereby facilitating rapid lead design and development.
AI Aids Pharmaceutical Manufacturing
AI also aids pharmaceutical manufacturing, as modern manufacturing systems attempt to impart human knowledge to machines, continually changing manufacturing practices. Applications of AI in manufacturing prove to be a push for the pharmaceutical industry. Tools like Computational Fluid Dynamics (CFD) use Reynolds-averaged Navier-Stokes solver techniques to study the effects of mixing and stress levels in different equipment, such as mixing tanks, automating pharmaceutical operations.
AI assists in quality control and assurance, from balancing various parameters in producing products from raw materials to maintaining batch-to-batch consistency requiring human intervention. The FDA has revised current Good Manufacturing Practices (cGMP), introducing a “quality by design” approach to understanding key operations and specific standards controlling drug final quality. AI can also regulate the online manufacturing process to meet product standards. Techniques like freeze-drying process monitoring based on artificial neural networks, combined with adaptive evolutionary algorithms, local search, and backpropagation algorithms, can predict future temperature and dried cake thickness under specific operational conditions, ultimately aiding in quality checks of final products. Furthermore, data mining and various knowledge discovery techniques in comprehensive quality management expert systems serve as valuable methods for making complex decisions, creating new technologies for intelligent quality control.
Prospects
Looking forward, AI advancements strive to alleviate the challenges faced by pharmaceutical companies, reshaping the drug development process and product lifecycle. This trend is evident in the burgeoning of startups in this sector. The healthcare industry grapples with complex challenges, such as the rising costs of drugs and treatments, requiring specific significant transformations in this field. With the application of AI in pharmaceutical product manufacturing, personalized medicines with required dosages and release parameters can be efficiently produced. Utilizing the latest AI-based technologies not only accelerates product time-to-market but also enhances product quality and safety, better utilizing existing resources while improving cost-effectiveness.
