The landscape of cancer treatment is undergoing a radical transformation, driven by the emergence of messenger RNA (mRNA) vaccines. These versatile and scalable platforms are not just a new tool; they represent a fundamental shift in how we approach immunotherapy. Unlike traditional vaccines that rely on attenuated or inactivated proteins, mRNA vaccines deliver genetic instructions directly to the body, teaching our own cells to produce specific antigens that can be recognized and targeted by the immune system. The astonishing success of mRNA technology during the COVID-19 pandemic has paved the way for its application in oncology, where it holds the promise of personalized, precise, and highly effective cancer treatment.
However, translating this promise into clinical reality requires overcoming significant challenges in the design and optimization of these vaccines. This is where the power of artificial intelligence (AI) and computational biology comes into play. By leveraging advanced computational tools, researchers can streamline every stage of the mRNA vaccine development process, from identifying the best targets to ensuring the vaccine’s stability and efficient delivery. This article explores the key areas where these technologies are revolutionizing mRNA vaccine design for cancer immunotherapy.
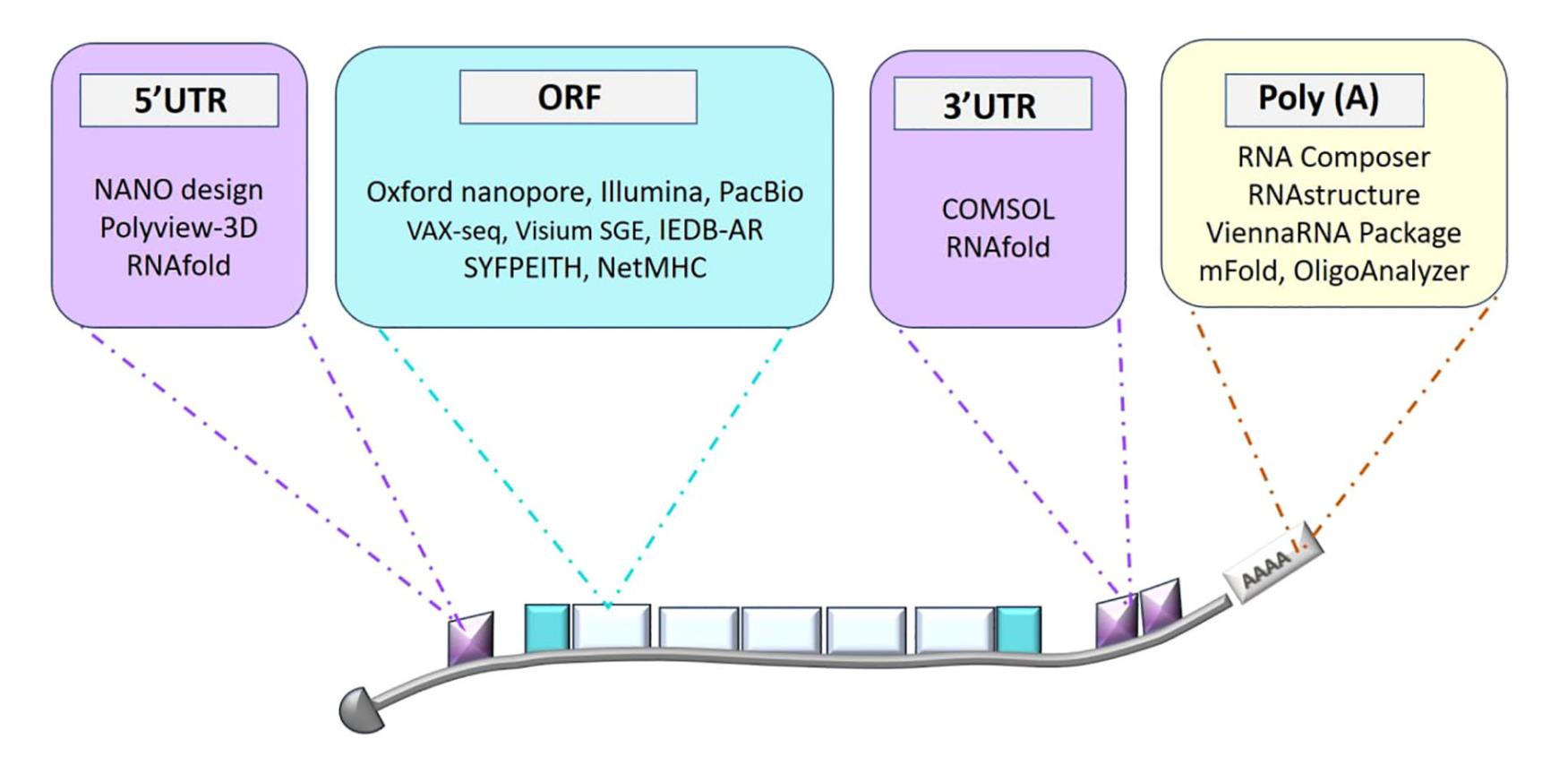
Pioneering Vaccine Design with Advanced Sequencing and Data Analysis
The journey of developing a personalized mRNA cancer vaccine begins with a deep dive into an individual’s unique genetic makeup. Next-generation sequencing (NGS) technologies, such as Illumina, Oxford Nanopore, and PacBio, are essential for this initial data acquisition. These platforms provide comprehensive genetic information on a patient’s tumor, allowing researchers to identify unique mutations and a specific type of antigen called neoantigens that are present on cancer cells but not on healthy cells.
- Illumina is a high-throughput technology that generates short, highly accurate sequence reads, crucial for identifying genetic variations in viral genomes.
- Oxford Nanopore offers real-time, long-read sequencing, which provides a complete view of full-length RNA transcripts and complex genomic regions. A key advantage is its ability to directly sequence mRNA without the need for reverse transcription, though its accuracy is currently moderate.
- PacBio also provides highly accurate long-read sequences, which are beneficial for detailed characterization and variant analysis of viral genomes.
Once the genetic data is acquired, a suite of bioinformatics tools is used to process and analyze it. Tools like
FASTQC and Trimmomatic are used for quality control, while alignment tools such as Burrows-Wheeler Aligner (BWA) and Bowtie align sequences to reference genomes to identify conserved regions and potential epitopes for the vaccine. This rigorous analysis is the foundation upon which the entire vaccine design process is built.
Precision Targeting: The Role of Antigen and Epitope Prediction
A major goal of personalized cancer immunotherapy is to design a vaccine that targets neoantigens with high precision, minimizing off-target effects and maximizing the therapeutic outcome. This requires accurately predicting which epitopes—the specific parts of an antigen that are recognized by the immune system—will trigger the strongest T-cell response.
Several powerful computational tools are used for this purpose:
- NetMHC predicts the binding affinity of peptides to Major Histocompatibility Complex (MHC) molecules, which are crucial for presenting antigens to T-cells. It has been updated with advanced algorithms, including artificial neural networks (ANNs), to improve its predictive capabilities, making it an essential resource for selecting optimal peptides for vaccine inclusion.
- The Immune Epitope Database Analysis Resource (IEDB-AR) is a comprehensive platform for predicting both T-cell and B-cell epitopes, making it ideal for designing vaccines against diseases with significant antigenic variation. It uses algorithms such as ANNs and Support Vector Machines (SVMs) to identify optimal antigenic targets that can stimulate both T-cell and antibody responses.
- SYFPEITHI is an older but still valuable tool that uses position-specific scoring matrices (PSSMs) to predict peptide-MHC interactions for both class I and II molecules, helping to pre-screen peptides and streamline the selection process for promising vaccine candidates.
By using these tools, researchers can efficiently select the most immunogenic epitopes, which is a crucial step in developing effective and specific mRNA vaccines.
Enhancing Vaccine Expression and Stability
Once a target epitope is identified, the next challenge is to design the mRNA sequence to ensure it is efficiently translated into the target protein and remains stable inside the body.
Codon optimization is a key step in this process. By modifying codons to match the host organism’s preferred profile, researchers can improve gene expression, reduce mRNA degradation, and enhance stability.
Tools like GeneOptimizer and JCAT (Java Codon Adaptation Tool) are used to achieve this optimization. GeneOptimizer uses a sliding window method to adjust codon usage and GC content, while JCAT uses algorithms like the Codon Adaptation Index (CAI) to align codon usage with host-specific tRNA pools. This process not only increases protein production but also enhances mRNA stability, leading to a stronger immune response.
In addition to codon optimization, predicting the mRNA’s secondary structure is vital for ensuring stability and translation efficiency. Tools like
RNAfold and mFold use thermodynamic models to predict the most stable secondary structures of RNA sequences. RNAfold, part of the Vienna RNA Package, provides detailed visualizations with base-pairing probabilities and positional entropy, offering a more in-depth analysis of structural stability. On the other hand, mFold offers quicker results and is useful for a more rapid, less detailed overview. Another specialized tool,
IPKnot, predicts complex structures, including pseudoknots, which are essential for understanding how mRNA folds and interacts with lipid nanoparticles (LNPs) and cellular machinery.
Optimizing Delivery and Function: The Crucial Role of LNPs
An mRNA vaccine is only as good as its delivery system. Lipid nanoparticles (LNPs) are the only FDA-approved carriers for mRNA vaccines because they effectively encapsulate and protect the fragile mRNA molecules, ensuring they are delivered efficiently to target cells. Computational tools are essential for designing and optimizing these complex structures.
- NANOdesign is a specialized tool for designing LNPs by allowing researchers to adjust lipid types, ratios, and particle size to achieve optimal mRNA encapsulation and stability.
- Molecular visualization tools like POLYVIEW-3D and PyMOL are used to create detailed 3D models of LNPs and visualize their interactions with mRNA and cell membranes at an atomic level. This helps researchers fine-tune lipid composition and surface properties for maximum delivery efficiency and stability.
Beyond design, molecular dynamics (MD) simulations and machine learning algorithms are used to optimize LNP formulations and predict their behavior in the body. MD simulation tools like
GROMACS and AMBER model the intricate movements and interactions of mRNA and LNP components, providing insights into their stability and function. For example, AMBER’s specialized force fields can accurately model nucleic acids and proteins to predict how mRNA vaccines will behave in a physiological environment.
The Power of AI in Fine-Tuning Vaccine Efficacy
The fusion of AI with computational biology is the next frontier in vaccine development. Machine learning algorithms, such as XGBoost, Graph Convolutional Networks (GCNs), and Deep Neural Networks (DNNs), are used to analyze vast datasets and make highly accurate predictions about vaccine efficacy and safety.
- XGBoost, combined with Bayesian optimization, can systematically evaluate a range of LNP formulation parameters with minimal experimental trials, accelerating the discovery of formulations that trigger strong immune responses.
- GCNs are uniquely suited to model the complex relationships within mRNA secondary structures and LNP formulations, guiding the design of more stable and efficient mRNA constructs.
- DNNs can analyze high-dimensional data, including lipid ratios, LNP size, and immunological endpoints, to predict how specific formulation changes will influence immune responses. This predictive capability is crucial for designing personalized vaccines tailored to an individual’s unique immune profile.
Future Prospects
The integration of AI and computational tools is not just improving existing processes; it is paving the way for a new era of personalized medicine and enhanced immunotherapy. By combining in silico simulations with multi-omics data (genomics, transcriptomics, proteomics, and metabolomics), researchers will be able to identify key biomarkers and pathways, leading to more targeted and effective vaccine strategies. This convergence of technology and biology promises to transform cancer treatment, offering hope for more precise and durable immune responses against one of the world’s most challenging diseases.
At Creative Biolabs, we are at the forefront of this revolution. Leveraging our expertise in AI and computational biology, we offer tailored services to accelerate your research and development efforts in this exciting field.
Reference:
1. Imani, Saber, et al. “Computational biology and artificial intelligence in mRNA vaccine design for cancer immunotherapy.” Frontiers in Cellular and Infection Microbiology 14 (2025): 1501010. Distributed under Open Access license CC BY 4.0, without modification.
